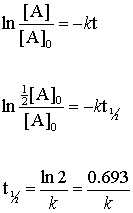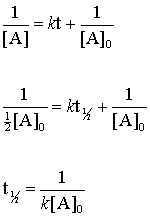half life formula for zero order reaction
From the above formula the half-life of the zero order kinetics depends on. In chemical kinetics the value of the half-life depends on the reaction order.
As long as you know 3 of the 4 values youll be able to use a half-life calculator.

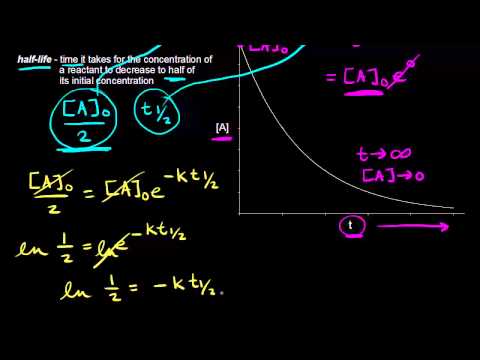
. For the first-order reaction the half-life is defined as t 1. Therefore A2 k 0 t ½ or t ½ A2k. I have used it every 3 days now for a total of 15 times.
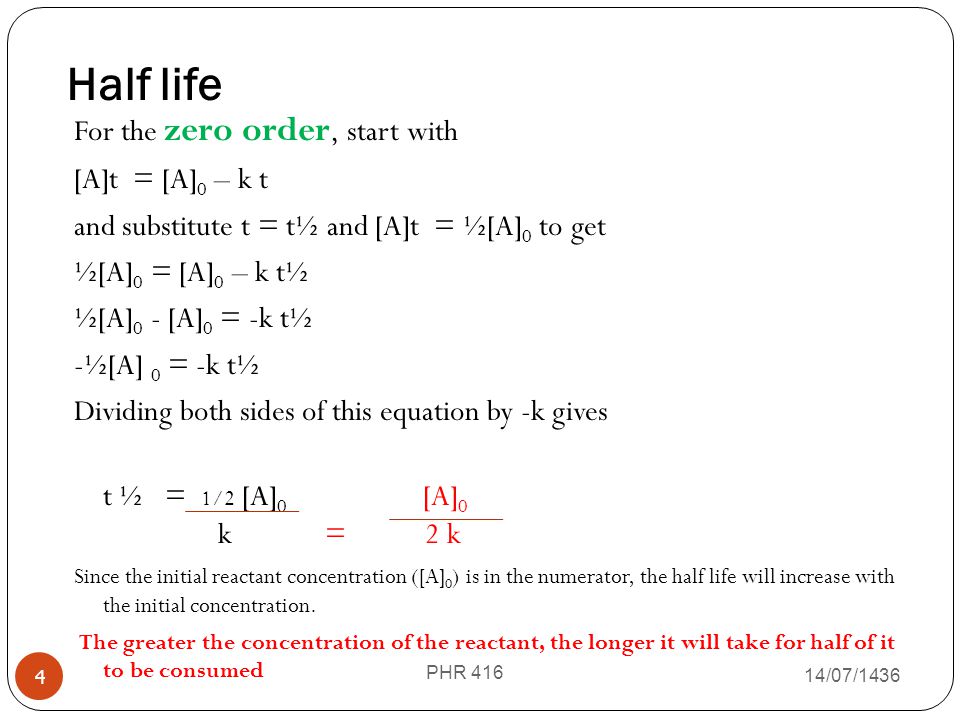
The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t 12 R 0 2k. When t t ½ that is the half-life of the reaction completed the concentration of the reactant A A2. No other reaction since.
In astronomy the main sequence is a continuous and distinctive band of stars that appears on plots of stellar color versus brightnessThese color-magnitude plots are known as HertzsprungRussell diagrams after their co-developers Ejnar Hertzsprung and Henry Norris RussellStars on this band are known as main-sequence stars or dwarf starsThese are the. Bond order can be calculated by the short cut method by the following formula rm Bond order 3 05rmn Here n is the difference between the total number of electrons and 14 in the given molecule. Knowledge of the reaction order quickly allows us to understand numerous factors within the reaction including the rate law units of the rate constant half life and much more.
The rate of this kind of reaction does not depend on the substrate concentration A. TextRate kAsBt label4 text. The half-life of a reaction is twice as long for a second-order reaction as it is for a first-order reaction.
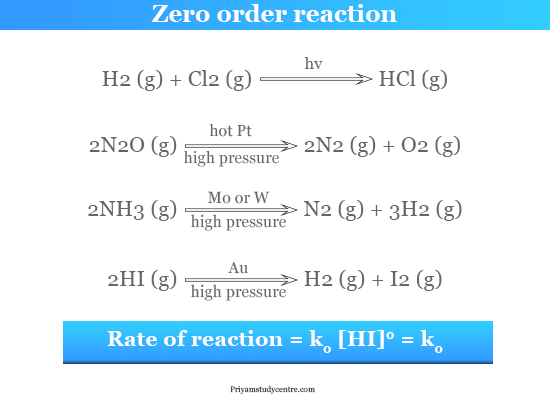
Half life in zero order reaction. For a zero-order reaction the mathematical expression that can be employed to determine the half-life is. The half-life formula for various reactions is given below.
Where ln2 is the natural logarithm of 2 approximately 0693. ZDNets technology experts deliver the best tech news and analysis on the latest issues and events in IT for business technology professionals IT managers and tech-savvy business people. Write a balanced half-reaction for the oxidation of liquid water H2O to gaseous oxygen O2 in acidic aqueous solution.
It is essential to note that the half-life formula of a reaction varies with the reactions order. My pores have almost disappeared giving me my 20 year old glowing skin I am almost 50 I only had a couple blemishes after first use. Looking for information on the anime ReZero kara Hajimeru Isekai Seikatsu ReZERO -Starting Life in Another World-.
Reaction order can be calculated from the rate law by adding the exponential values of the reactants in the rate law. My makeup goes on smooth again with zero lines or cracking. Write the formula to calculate bond order of molecule.
The molecular oxygen involved in this reaction generally goes from its oxidation state of zero to a lower oxidation state of -2 whereas the substance which is undergoing combustion gains energy from the heat energy being supplied to it for the process of combustion and goes from its lower oxidation state its original form to a. For a first-order reaction the half-life is given by. T 12 0693k.
Formula through a program called WIC for low-income mothers infants and children. 112 Half-life and reaction orders. What is the shortest trick to calculate bond order.
Then you can use any half-life calculator online to determine the half-life. Be sure to add physical state symbols where appropriate. Those companies also dominate federal contracts that provide about half of all US.
GCSE Biology revision Cell structure Cell division Transport in cells Digestive system Heart and blood Health issues Plant tissues organs and systems Communicable diseases Drugs Plant disease Photosynthesis Respiration Homeostasis Nervous system Hormones Reproduction Variation and Evolution Ecosystems Biodiversity Trophic levels Food production GCSE. T 12 R 0 2k. View Answer Iodine-131 has a half-life of 870 days.
If you know the half-life but you dont know the initial quantity you can input the half-life the quantity that remains and the time that has passed. T 12 is the half-life units. When Subaru Natsuki leaves the convenience store the last thing he expects is to be wrenched from his everyday life and.
It is important to note that the formula for the half-life of a reaction varies with the order of the reaction. Half life means 50 percent of reactants disappear in that time interval. Find out more with MyAnimeList the worlds most active online anime and manga community and database.
This formula for the magnetic force on a current carrying wire is the basis for the experiment that was used to define the ampère from 1948 to 2019. The general formula for these materials is A x PRCN 6 and their crystal structure is analogous to that of the ABX 3 perovskites with P m and R. Zero-order reaction has successive half-lives which decrease with time First-order reaction has a constant half-life where half-life is independent to the concentration Second-order reaction has successive half-lives which increase with time Calculating k from half-life first-order reactions.

Zero Order Reactions Chemistry Class 12 Iit Jee Main Advanced Neet Aipmt Askiitians Youtube

Zero Order Reactions Video Kinetics Khan Academy
Zero Order Reaction Definition Examples Formula
Kinetics Order Of Reactions Ppt Video Online Download

Half Life Of Zero Th 0th Order Reaction Derivation Youtube

Half Life Expressions Chemistnate

Half Life Expressions Chemistnate

Zero Order Reaction Definition Examples Formula

Integrated Rate Laws Chemistry For Majors
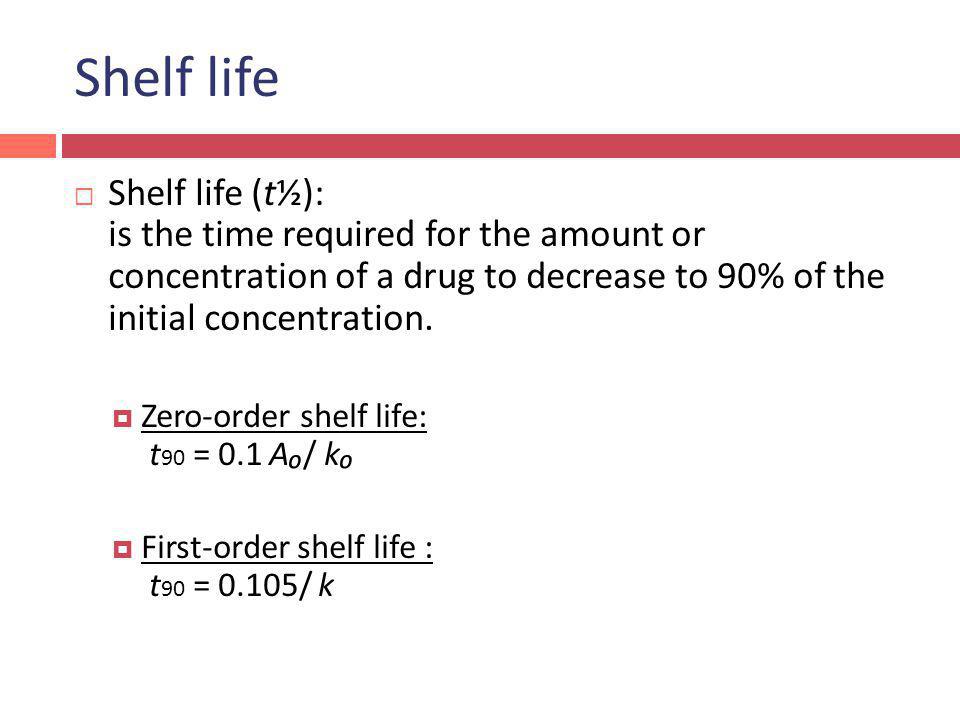
Principles And Kinetics Of Drug Stability Phr 416 Ppt Video Online Download

Half Life Expressions Chemistnate

Integrated Rate Laws Chemistry For Majors

Which Of The Following Statements Are Corrects
Half Life Of A First Order Reaction Video Khan Academy

Derive The Integrated Half Life Equation For Zero Order Reaction Chemistry Chemical Kinetics 12889537 Meritnation Com